Arylcyclohexylamine
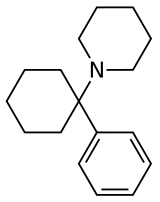
Arylcyclohexylamines, also known as arylcyclohexamines or arylcyclohexanamines, are a chemical class of pharmaceutical, designer, and experimental drugs.
History[]
Phencyclidine (PCP) is believed to be the first arylcyclohexylamine with recognized anesthetic properties, but several arylcyclohexylamines were described before PCP in the scientific literature, beginning with PCA (1-phenylcyclohexan-1-amine) the synthesis of which was first published in 1907. PCE was reported in 1953 and PCMo (4-(1-phenyl-cyclohexyl)-morpholine[1] see chart below for figure) in 1954, with PCMo described as a potent sedative.[2] Arylcyclohexylamine anesthetics were intensively investigated at Parke-Davis, beginning with the 1956 synthesis of phencyclidine and later the related compound ketamine.[2] The 1970s saw the debut of these compounds, especially PCP and its analogues, as illicitly used recreational drugs due to their dissociative hallucinogenic and euphoriant effects. Since that time, the class has been expanded by scientific research into stimulant, analgesic, and neuroprotective agents, and also by clandestine chemists in search of novel recreational drugs.[3][4][5]
Structure[]

An arylcyclohexylamine is composed of a cyclohexylamine unit with an aryl moiety attachment. The aryl group is positioned geminal to the amine. In the simplest cases, the aryl moiety is typically a phenyl ring, sometimes with additional substitution. The amine is usually not primary; secondary amines such as methylamino or ethylamino, or tertiary cycloalkylamines such as piperidino and pyrrolidino, are the most commonly encountered N-substituents.
Pharmacology[]
Arylcyclohexylamines varyingly possess NMDA receptor antagonistic,[6][7] dopamine reuptake inhibitory,[8] and μ-opioid receptor agonistic[9] properties. Additionally, σ receptor agonistic,[10] nACh receptor antagonistic,[11] and D2 receptor agonistic[12] actions have been reported for some of these agents. Antagonism of the NMDA receptor confers anesthetic, anticonvulsant, neuroprotective, and dissociative effects; blockade of the dopamine transporter mediates stimulant and euphoriant effects as well as psychosis in high amounts; and activation of the μ-opioid receptor causes analgesic and euphoriant effects. Stimulation of the σ and D2 receptors may also contribute to hallucinogenic and psychotomimetic effects.[12]
These are versatile agents with a wide range of possible pharmacological activities depending on the extent and range to which chemical modifications are implemented.[13][14][15][16][17][18][19][20][21] The various choice of substitutions that are made allows for "fine-tuning" of the pharmacological profile that results. As examples, BTCP is a selective dopamine reuptake inhibitor,[8] PCP is primarily an NMDA antagonist,[6] and BDPC is a potent μ-opioid agonist,[22] while PRE-084 is a selective sigma receptor agonist.[23] Thus, radically different pharmacology is possible through different structural combinations.
Notes on numbering[]
PCP itself is composed of three six-membered rings, which can each be substituted by a variety of groups. These are traditionally numbered in the older research as first the cyclohexyl ring, then the phenyl, and finally the piperidine ring, with the different rings represented by prime notation (') next to the number. For instance, 4-methyl-PCP, 4'-methyl-PCP and 4''-methyl-PCP are all known compounds, with similar activity but quite different potencies.

However, since the widespread sale of these compounds as grey-market designer drugs, nearly all such compounds that have come to prominence either have a bare cyclohexyl ring or a 2-ketocyclohexyl ring, while the piperidine is replaced by a variety of alkyl or cycloalkyl amines and most substitution has taken place on the phenyl ring. Consequently it is common for widely used phenyl substituted analogues such as 3'-MeO-PCP and 3'-MeO-PCE to be referred to as 3-MeO-PCP and 3-MeO-PCE without the prime, even though this is technically incorrect and could lead to confusion.
List of arylcyclohexylamines[]
| Structure | Compound | Aryl Substituent | N Group | Cyclohexyl ring | CAS number |
|---|---|---|---|---|---|
 |
PCA[24] | Phenyl | NH2 | - | 1934-71-0 |
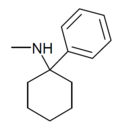 |
PCM[24] | Phenyl | Methylamino | - | 2201-16-3 |
 |
Eticyclidine | Phenyl | Ethylamino | - | 2201-15-2 |
 |
PCPr[25] | Phenyl | n-Propylamino | - | 18949-81-0 |
 |
PCiP | Phenyl | Isopropylamino | - | 1195-42-2 |
 |
PCAL [26] | Phenyl | Allylamino | - | 2185-95-7 |
 |
PCBu | Phenyl | n-Butylamino | - | 73166-29-7 |
 |
PCEOH | Phenyl | Hydroxyethylamino | - | 2201-22-1 |
 |
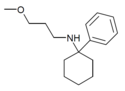
PCMEA[27] | Phenyl | Methoxyethylamino | - | 2201-57-2 |
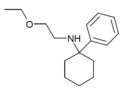 |
PCEEA | Phenyl | Ethoxyethylamino | - | 1072895-05-6 |
 |
PCMPA | Phenyl | Methoxypropylamino | - | 2201-58-3 |
 |
PCDM[24] | Phenyl | Dimethylamino | - | 2201-17-4 |
 |
Dieticyclidine | Phenyl | Diethylamino | - | 2201-19-6 |
 |
2-HO-PCP[6] | Phenyl | Piperidine | 2-Hydroxy | 94852-58-1 |
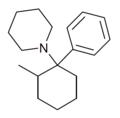 |
2-Me-PCP[28] | Phenyl | Piperidine | 2-Methyl | 59397-29-4 |
 |
2-MeO-PCP[29] | Phenyl | Piperidine | 2-Methoxy | 78636-34-7 |
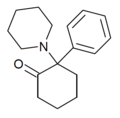 |
2-Keto-PCP | Phenyl | Piperidine | 2-Keto | 101688-16-8 |
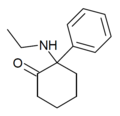 |
Eticyclidone ("O-PCE") | Phenyl | Ethylamino | 2-Keto | 6740-82-5 |
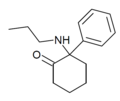 |
2-Keto-PCPr | Phenyl | n-Propylamino | 2-Keto | |
 |
4-Methyl-PCP | Phenyl | Piperidine | 4-Methyl | 19420-52-1 |
 |
4-Keto-PCP[30] | Phenyl | Piperidine | 4-Keto | 65620-13-5 |
 |
2'-Cl-PCP | o-Chlorophenyl | Piperidine | - | 2201-31-2 |
 |
3'-Cl-PCP | m-Chlorophenyl | Piperidine | - | 2201-32-3 |
 |
2'-MeO-PCP | o-Methoxyphenyl | Piperidine | - | 2201-34-5 |
 |
3'-F-PCP[31] | m-Fluorophenyl | Piperidine | - | 89156-99-0 |
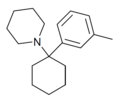 |
3'-Me-PCP[32] | m-Tolyl | Piperidine | - | 2201-30-1 |
 |
3'-Me-PCPy | m-Tolyl | Pyrrolidine | - | 1622348-63-3 |
 |
3'-NH2-PCP | m-Aminophenyl | Piperidine | - | 72242-00-3 |
 |
3'-HO-PCP | m-Hydroxyphenyl | Piperidine | - | 79787-43-2 |
 |
3'-MeO-PCP | m-Methoxyphenyl | Piperidine | - | 72242-03-6 |
 |
3',4'-MD-PCP | 3,4-Methylenedioxyphenyl | Piperidine | - | |
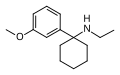 |
3'-MeO-PCE | m-Methoxyphenyl | Ethylamino | - | 1364933-80-1 |
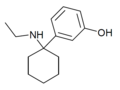 |
3'-HO-PCE | m-Hydroxyphenyl | Ethylamino | - | |
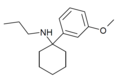 |
3'-MeO-PCPr | m-Methoxyphenyl | n-Propylamino | - | 1364933-81-2 |
 |
3'-HO-PCPr | m-Hydroxyphenyl | n-Propylamino | - | |
 |
3',4'-MD-PCPr | 3,4-Methylenedioxyphenyl | n-Propylamino | - | |
 |
3'-MeO-PCPy[32] | m-Methoxyphenyl | Pyrrolidine | - | 1364933-79-8 |
 |
4'-HO-PCP | p-Hydroxyphenyl | Piperidine | - | 66568-88-5 |
 |
Methoxydine (4'-MeO-PCP) | p-Methoxyphenyl | Piperidine | - | 2201-35-6 |
 |
4'-MeO-PCE | p-Methoxyphenyl | Ethylamino | - | |
 |
4'-F-PCP[31] | p-Fluorophenyl | Piperidine | - | 22904-99-0 |
 |
4'-F-PCPy | p-Fluorophenyl | Pyrrolidine | - | |
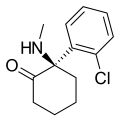 |
Arketamine | o-Chlorophenyl | Methylamino | 2-Keto | 33643-49-1 |
 |
Deschloroketamine | Phenyl | Methylamino | 2-Keto | 7063-30-1 |
 |
Esketamine | o-Chlorophenyl | Methylamino | 2-Keto | 33643-46-8 |
 |
Ketamine | o-Chlorophenyl | Methylamino | 2-Keto | 6740-88-1 |
 |
Hydroxynorketamine | o-Chlorophenyl | NH2 | 2-Keto, 6-Hydroxy | 81395-70-2 |
 |
Ethketamine | o-Chlorophenyl | Ethylamino | 2-Keto | 1354634-10-8 |
 |
NPNK | o-Chlorophenyl | n-Propylamino | 2-Keto | |
 |
Methoxyketamine | o-Methoxyphenyl | Methylamino | 2-Keto | 7063-51-6 |
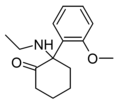 |
2-MeO-NEK[33] | o-Methoxyphenyl | Ethylamino | 2-Keto | |
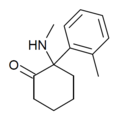 |
oMDCK | o-Tolyl | Methylamino | 2-Keto | 7063-37-8 |
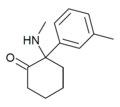 |
mMDCK | m-Tolyl | Methylamino | 2-Keto | |
 |
meta-Ketamine | m-Chlorophenyl | Methylamino | 2-Keto | 7063-53-8 |
 |
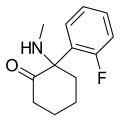
iso-Ketamine | o-Chlorophenyl | Methylamino | 4-Keto | |
 |
2-Fluorodeschloroketamine | o-Fluorophenyl | Methylamino | 2-Keto | 111982-50-4 |
 |
3-Fluorodeschloroketamine | m-Fluorophenyl | Methylamino | 2-Keto | |
 |
Bromoketamine | o-Bromophenyl | Methylamino | 2-Keto | 120807-70-7 |
 |
TFMDCK | o-Trifluoromethylphenyl | Methylamino | 2-Keto | 1782149-73-8 |
 |
SN 35210[34] | o-Chlorophenyl | Carbomethoxybutylamino | 2-Keto | 1450615-54-9 |
 |
Methoxetamine | m-Methoxyphenyl | Ethylamino | 2-Keto | 1239943-76-0 |
 |
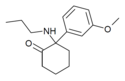
Methoxmetamine | m-Methoxyphenyl | Methylamino | 2-Keto | 1781829-56-8 |
 |
Methoxpropamine | m-Methoxyphenyl | n-Propylamino | 2-Keto | 2504100-71-2 |
 |
MXiPr | m-Methoxyphenyl | i-Propylamino | 2-Keto | |
 |
Ethoxetamine | m-Ethoxyphenyl | Ethylamino | 2-Keto | |
 |
Deoxymethoxetamine (3-Me-2'-Oxo-PCE) | m-Tolyl | Ethylamino | 2-Keto | |
 |
Br-MXE | 2-bromo-5-methoxyphenyl | Ethylamino | 2-Keto | |
 |
Hydroxetamine (HXE) | m-Hydroxyphenyl | Ethylamino | 2-Keto | 1620054-73-0 |
 |
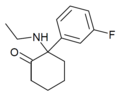
HXM | m-Hydroxyphenyl | Methylamino | 2-Keto | |
 |
Fluorexetamine (FXE) | m-Fluorophenyl | Ethylamino | 2-Keto | |
 |
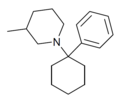
Phencyclidine (PCP) | Phenyl | Piperidine | - | 77-10-1 |
 |
PC3MP | Phenyl | 3-Methylpiperidine | - | 2201-41-4 |
 |
PC4MP | Phenyl | 4-Methylpiperidine | - | 2201-42-5 |
 |
Rolicyclidine (PCPy) | Phenyl | Pyrrolidine | - | 2201-39-0 |
 |
PCDMPy | Phenyl | 3,3-Dimethylpyrrolidine | - | |
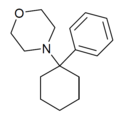 |
PCMo | Phenyl | Morpholine | - | 2201-40-3 |
 |
Methoxy-PCM[7] (2'-MeO-PCMo) | o-Methoxyphenyl | Morpholine | - | 1314323-88-0 |
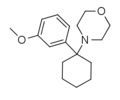 |
3'-MeO-PCMo | m-Methoxyphenyl | Morpholine | - | 138873-80-0 |
 |
4'-MeO-PCMo | p-Methoxyphenyl | Morpholine | - | |
 |
Methyl-PCM[35] (4'-Me-PCMo) | p-Tolyl | Morpholine | - | 120803-52-3 |
 |
Hydroxy-methyl-PCM | 2-Methyl-4-hydroxyphenyl | Morpholine | - | 1314323-89-1 |
 |
PYCP [36] | 2-Pyridinyl | Piperidine | - | |
 |
TCM | 2-Thienyl | Methylamino | - | 139401-07-3 |
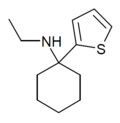 |
TCE | 2-Thienyl | Ethylamino | - | 101589-62-2 |
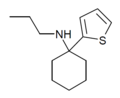 |
TCPr [37] | 2-Thienyl | Propylamino | - | |
 |
Tenocyclidine (TCP) | 2-Thienyl | Piperidine | - | 21500-98-1 |
 |
T3CP | 3-Thienyl | Piperidine | - | 19420-50-9 |
 |
TCPy | 2-Thienyl | Pyrrolidine | - | 22912-13-6 |
 |
Tiletamine | 2-Thienyl | Ethylamino | 2-Keto | 14176-49-9 |
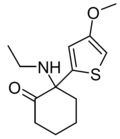 |
MXTE | 4-Methoxy-2-thienyl | Ethylamino | 2-Keto | |
 |
Gacyclidine | 2-Thienyl | Piperidine | 2-Methyl | 68134-81-6 |
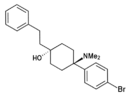 |
BDPC | p-Bromophenyl | Dimethylamino | 4-Phenethyl-4-hydroxy | 77239-98-6 |
 |
C-8813 | p-Bromophenyl | Dimethylamino | 4-(thiophen-2-yl)ethyl-4-hydroxy | 616898-54-5 |
 |
Dimetamine[38] | p-Tolyl | Dimethylamino | 4-Keto | 65619-06-9 |
 |
3''-OH-2'-Me-PCP [39] | o-Tolyl | 3-Hydroxypiperidine | - | |
 |
4''-Ph-4''-OH-PCP [40] | Phenyl | 4-Phenyl-4-hydroxypiperidine | - | 77179-39-6 |
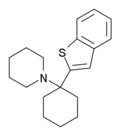 |
BTCP[41] | Benzothiophen-2-yl | Piperidine | - | 112726-66-6 |
 |
BTCPy[10] | Benzothiophen-2-yl | Pyrrolidine | - | |
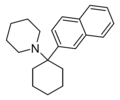 |
GK-189[42] | Naphthalen-2-yl | Piperidine | - | 81490-58-6 |
Related compounds[]
Other similar compounds exist where the base ring has been varied, or the amine chain replaced with other groups.[43] More cycloalkane ring sizes have been experimented with than just purely thinking in terms of the cyclohexylamine. The cyclopentyl homologue of PCP is active with around 1/10th the potency,[44] while the cycloheptyl and cyclooctyl derivatives are inactive, though some substituted arylcycloheptylamines retain activity.[45] The requisite cycloalkylketone is reacted with PhMgBr; 3° alcohol is then reacted with NaN3; azide then reduced with LAH. Then in the final step the piperidine ring is constructed with 1-5-dibromo-pentane.[46] Other compounds are known where the cyclohexyl base ring is replaced by rings such as norbornyl, adamantyl,[47] tetralin, oxane or piperidine.[48] Conformationally constrained analogs have been prepared and researched by Morieti et al.[49]
| Structure | Compound | Aryl Substituent | N Group | Base ring | CAS number |
|---|---|---|---|---|---|
 |
PCPEP | Phenyl | Piperidine | Cyclopentyl | 23036-19-3 |
 |
3F-PCHEPy | 3-Fluorophenyl | Pyrrolidine | Cycloheptyl | |
 |
3-MeO-PBCHP | 3-Methoxyphenyl | Piperidine | Bicyclo[2.2.1]heptane | |
 |
PADP | Phenyl | Piperidine | Adamantyl | 72241-99-7 |
 |
3-MeO-PTP | 3-Methoxyphenyl | Piperidine | Tetralin | |
 |
HHFA | Fused phenyl | Amino | Hexahydrofluorene | |
 |
DHPQ | Phenyl | Decahydroquinoline | ||
 |
POXP | Phenyl | Piperidine | Oxane | |
 |
MPBPip | Phenyl | Piperidine | N-Methylpiperidine | 36882-04-9 |
 |
BnCP | Benzyl | Piperidine | Cyclohexyl | 22912-07-8 |
 |
YNCP | Ethynyl | Piperidine | Cyclohexyl | 51165-02-7 |
 |
ALCP | Allyl | Piperidine | Cyclohexyl | 7418-80-6 |
 |
Piritramide | Replaced by carboxamide | Piperidine | N-(3-cyano-3,3-diphenylpropyl)piperidine | 302-41-0 |
 |
PRE-084 | Phenyl | Morpholinylethylcarboxylate | Cyclohexyl | 138847-85-5 |
 |
Clofenciclan | p-Chlorophenyl | Diethylaminoethoxy | Cyclohexyl | 5632-52-0 |
References[]
- ^ "4-(1-phenyl-cyclohexyl)-morpholine". CAS Number Search - chemsrc.com. chemsrc. Retrieved 15 March 2021.
- ^ a b Morris H, Wallach J (2014). "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis. 6 (7–8): 614–32. doi:10.1002/dta.1620. PMID 24678061.
- ^ Valter K, Arrizabalaga P (1998). Designer Drugs Directory. Elsevier. ISBN 0-444-20525-X.
- ^ Wallach J, Brandt SD (August 2018). "Phencyclidine-Based New Psychoactive Substances". New Psychoactive Substances. Handbook of Experimental Pharmacology. Vol. 252. pp. 261–303. doi:10.1007/164_2018_124. ISBN 978-3-030-10560-0. PMID 30105474.
- ^ Wallach J, Brandt SD (2018). "1,2-Diarylethylamine- and Ketamine-Based New Psychoactive Substances". Handbook of Experimental Pharmacology. 252: 305–352. doi:10.1007/164_2018_148. ISBN 978-3-030-10560-0. PMID 30196446.
- ^ a b c Ahmadi A, Mahmoudi A (2005). "Synthesis and biological properties of 2-hydroxy-1-(1-phenyltetralyl)piperidine and some of its intermediates as derivatives of phencyclidine". Arzneimittel-Forschung. 55 (9): 528–32. doi:10.1055/s-0031-1296900. PMID 16229117.
- ^ a b Ahmadi A, Khalili M, Hajikhani R, Naserbakht M (April 2011). "New morpholine analogues of phencyclidine: chemical synthesis and pain perception in rats". Pharmacology, Biochemistry, and Behavior. 98 (2): 227–33. doi:10.1016/j.pbb.2010.12.019. PMID 21215770. S2CID 24650035.
- ^ a b Chaudieu I, Vignon J, Chicheportiche M, Kamenka JM, Trouiller G, Chicheportiche R (March 1989). "Role of the aromatic group in the inhibition of phencyclidine binding and dopamine uptake by PCP analogs". Pharmacology, Biochemistry, and Behavior. 32 (3): 699–705. doi:10.1016/0091-3057(89)90020-8. PMID 2544905. S2CID 7672918.
- ^ Itzhak Y, Simon EJ (August 1984). "A novel phencyclidine analog interacts selectively with mu opioid receptors". The Journal of Pharmacology and Experimental Therapeutics. 230 (2): 383–6. PMID 6086884.
- ^ a b He XS, Raymon LP, Mattson MV, Eldefrawi ME, de Costa BR (April 1993). "Synthesis and biological evaluation of 1-[1-(2-benzo[b]thienyl)cyclohexyl]piperidine homologues at dopamine-uptake and phencyclidine- and sigma-binding sites". Journal of Medicinal Chemistry. 36 (9): 1188–93. doi:10.1021/jm00061a009. PMID 8098066.
- ^ Eterović VA, Lu R, Eakin AE, Rodríguez AD, Ferchmin PA (December 1999). "Determinants of phencyclidine potency on the nicotinic acetylcholine receptors from muscle and electric organ". Cellular and Molecular Neurobiology. 19 (6): 745–57. doi:10.1023/A:1006905106834. PMID 10456235. S2CID 22266452.
- ^ a b Seeman P, Ko F, Tallerico T (September 2005). "Dopamine receptor contribution to the action of PCP, LSD and ketamine psychotomimetics". Molecular Psychiatry. 10 (9): 877–83. doi:10.1038/sj.mp.4001682. PMID 15852061.
- ^ al-Deeb OA (May 1996). "New analgesics derived from the phencyclidine analogue thienylcyclidine". Arzneimittel-Forschung. 46 (5): 505–8. PMID 8737636.
- ^ Ahmadi A, Khalili M, Hajikhani R, Hosseini H, Afshin N, Nahri-Niknafs B (March 2012). "Synthesis and study the analgesic effects of new analogues of ketamine on female wistar rats". Medicinal Chemistry. 8 (2): 246–51. doi:10.2174/157340612800493683. PMID 22385170. S2CID 42842315.
- ^ Ahmadi A, Khalili M, Abbassi S, Javadi M, Mahmoudi A, Hajikhani R (2009). "Synthesis and study on analgesic effects of 1-[1-(4-methylphenyl) (cyclohexyl)] 4-piperidinol and 1-[1-(4-methoxyphenyl) (cyclohexyl)] 4-piperidinol as two new phencyclidine derivatives". Arzneimittel-Forschung. 59 (4): 202–6. doi:10.1055/s-0031-1296386. PMID 19517897. S2CID 5402425.
- ^ Ahmadi A, Khalili M, Marami S, Ghadiri A, Nahri-Niknafs B (January 2014). "Synthesis and pain perception of new analogues of phencyclidine in NMRI male mice". Mini Reviews in Medicinal Chemistry. 14 (1): 64–71. doi:10.2174/1389557513666131119203551. PMID 24251803.
- ^ Ahmadi A, Solati J, Hajikhani R, Pakzad S (2011). "Synthesis and analgesic effects of new pyrrole derivatives of phencyclidine in mice". Arzneimittel-Forschung. 61 (5): 296–300. doi:10.1055/s-0031-1296202. PMID 21755813. S2CID 24287727.
- ^ Ahmadi A, Khalili M, Hajikhani R, Barghi L, Mihandoust F (2010). "Synthesis and determination of chronic and acute thermal and chemical pain activities of a new derivative of phencyclidine in rats". Iranian Journal of Pharmaceutical Research. 9 (4): 379–85. PMC 3870061. PMID 24381602.
- ^ Ahmadi A, Khalili M, Mihandoust F, Barghi L (2010). "Synthesis and determination of acute and chronic pain activities of 1-[1-(3-methylphenyl) (tetralyl)]piperidine as a new derivative of phencyclidine via tail immersion and formalin tests". Arzneimittel-Forschung. 60 (1): 30–5. doi:10.1055/s-0031-1296245. PMID 20184224. S2CID 23966936.
- ^ Hajikhani R, Ahmadi A, Naderi N, Yaghoobi K, Shirazizand Z, Rezaee NM, Niknafs BN (2012). "Effect of phencyclidine derivatives on anxiety-like behavior using an elevated-plus maze test in mice". Advances in Clinical and Experimental Medicine. 21 (3): 307–12. PMID 23214193.
- ^ Ahmadi A, Khalili M, Mirza B, Mohammadi-Diz M, Azami-Lorestani F, Ghaderi P, Nahri-Niknafs B (2017). "Synthesis and Antinociception Activities of Some Novel Derivatives of Phencyclidine with Substituted Aminobenzothiazoles". Mini Reviews in Medicinal Chemistry. 17 (1): 78–84. doi:10.2174/1389557516666160428112532. PMID 27121715.
- ^ Lednicer D, VonVoigtlander PF (October 1979). "4-(p-Bromophenyl)-4-(dimethylamino)-1-phenethylcyclohexanol, an extremely potent respresentative of a new analgesic series". Journal of Medicinal Chemistry. 22 (10): 1157–8. doi:10.1021/jm00196a001. PMID 513062.
- ^ Maurice T, Su TP, Parish DW, Nabeshima T, Privat A (December 1994). "PRE-084, a sigma selective PCP derivative, attenuates MK-801-induced impairment of learning in mice". Pharmacology, Biochemistry, and Behavior. 49 (4): 859–69. doi:10.1016/0091-3057(94)90235-6. PMID 7886099. S2CID 54306053.
- ^ a b c Thurkauf A, de Costa B, Yamaguchi S, Mattson MV, Jacobson AE, Rice KC, Rogawski MA (May 1990). "Synthesis and anticonvulsant activity of 1-phenylcyclohexylamine analogues". Journal of Medicinal Chemistry. 33 (5): 1452–8. doi:10.1021/jm00167a027. PMID 2329567.
- ^ Sauer C, Peters FT, Staack RF, Fritschi G, Maurer HH (April 2008). "Metabolism and toxicological detection of a new designer drug, N-(1-phenylcyclohexyl)propanamine, in rat urine using gas chromatography-mass spectrometry". Journal of Chromatography A. 1186 (1–2): 380–90. doi:10.1016/j.chroma.2007.11.002. PMID 18035363.
- ^ Kalir A, Teomy S, Amir A, Fuchs P, Lee SA, Holsztynska EJ, et al. (October 1984). "N-allyl analogues of phencyclidine: chemical synthesis and pharmacological properties". Journal of Medicinal Chemistry. 27 (10): 1267–71. doi:10.1021/jm00376a006. PMID 6481761.
- ^ Sauer C, Peters FT, Schwaninger AE, Meyer MR, Maurer HH (February 2009). "Investigations on the cytochrome P450 (CYP) isoenzymes involved in the metabolism of the designer drugs N-(1-phenyl cyclohexyl)-2-ethoxyethanamine and N-(1-phenylcyclohexyl)-2-methoxyethanamine". Biochemical Pharmacology. 77 (3): 444–50. doi:10.1016/j.bcp.2008.10.024. PMID 19022226.
- ^ Iorio MA, Tomassini L, Mattson MV, George C, Jacobson AE (August 1991). "Synthesis, stereochemistry, and biological activity of the 1-(1-phenyl-2-methylcyclohexyl)piperidines and the 1-(1-phenyl-4-methylcyclohexyl)piperidines. Absolute configuration of the potent trans-(-)-1-(1-phenyl-2-methylcyclohexyl)piperidine". Journal of Medicinal Chemistry. 34 (8): 2615–23. doi:10.1021/jm00112a041. PMID 1875352.
- ^ Ahmadi A, Mahmoudi A (2006). "Synthesis with improved yield and study on the analgesic effect of 2-methoxyphencyclidine". Arzneimittel-Forschung. 56 (5): 346–50. doi:10.1055/s-0031-1296732. PMID 16821645.
- ^ Ortiz DM, Custodio RJ, Abiero A, Botanas CJ, Sayson LV, Kim M, et al. (July 2021). "The dopaminergic alterations induced by 4-F-PCP and 4-Keto-PCP may enhance their drug-induced rewarding and reinforcing effects: Implications for abuse". Addiction Biology. 26 (4): e12981. doi:10.1111/adb.12981. PMID 33135332. S2CID 226234538.
- ^ a b Ogunbadeniyi AM, Adejare A (2002). "Syntheses of fluorinated phencyclidine analogs". Journal of Fluorine Chemistry. 114: 39–42. doi:10.1016/S0022-1139(01)00565-6.
- ^ a b Wallach J, De Paoli G, Adejare A, Brandt SD (2013). "Preparation and analytical characterization of 1-(1-phenylcyclohexyl)piperidine (PCP) and 1-(1-phenylcyclohexyl)pyrrolidine (PCPy) analogues". Drug Testing and Analysis. 6 (7–8): 633–50. doi:10.1002/dta.1468. PMID 23554350.
- ^ Sayson LV, Botanas CJ, Custodio RJ, Abiero A, Kim M, Lee HJ, et al. (July 2019). "The novel methoxetamine analogs N-ethylnorketamine hydrochloride (NENK), 2-MeO-N-ethylketamine hydrochloride (2-MeO-NEK), and 4-MeO-N-ethylketamine hydrochloride (4-MeO-NEK) elicit rapid antidepressant effects via activation of AMPA and 5-HT2 receptors". Psychopharmacology. 236 (7): 2201–2210. doi:10.1007/s00213-019-05219-x. PMID 30891619. S2CID 83463722.
- ^ Harvey M, Sleigh J, Voss L, Pruijn F, Jose J, Gamage S, Denny W (2015). "Determination of the Hypnotic Potency in Rats of the Novel Ketamine Ester Analogue SN 35210". Pharmacology. 96 (5–6): 226–32. doi:10.1159/000439598. PMID 26352278. S2CID 36017002.
- ^ Ahmadi A, Khalili M, Hajikhani R, Naserbakht M (2011). "Synthesis and determination of acute and chronic pain activities of 1-[1-(4-methylphenyl) (cyclohexyl)] morpholine as a new phencyclidine derivative in rats". Arzneimittel-Forschung. 61 (2): 92–7. doi:10.1055/s-0031-1296173. PMID 21428243.
- ^ Zarantonello P, Bettini E, Paio A, Simoncelli C, Terreni S, Cardullo F (April 2011). "Novel analogues of ketamine and phencyclidine as NMDA receptor antagonists". Bioorganic & Medicinal Chemistry Letters. 21 (7): 2059–63. doi:10.1016/j.bmcl.2011.02.009. PMID 21334205.
- ^ Wallach J, Colestock T, Cicali B, Elliott SP, Kavanagh PV, Adejare A, et al. (August 2016). "Syntheses and analytical characterizations of N-alkyl-arylcyclohexylamines" (PDF). Drug Testing and Analysis. 8 (8): 801–15. doi:10.1002/dta.1861. PMID 26360516.
- ^ Lednicer D, VonVoigtlander PF, Emmert DE (April 1980). "4-Amino-4-arylcyclohexanones and their derivatives, a novel class of analgesics. 1. Modification of the aryl ring". Journal of Medicinal Chemistry. 23 (4): 424–30. doi:10.1021/jm00178a014. PMID 7381841.
- ^ Ahmadi A, Solati J, Hajikhani R, Onagh M, Javadi M (2010). "Synthesis and analgesic effects of 1-[1-(2-methylphenyl)(cyclohexyl)]-3-piperidinol as a new derivative of phencyclidine in mice". Arzneimittel-Forschung. 60 (8): 492–6. doi:10.1055/s-0031-1296317. PMID 20863005. S2CID 24803623.
- ^ Itzhak Y, Kalir A, Weissman BA, Cohen S (May 1981). "New analgesic drugs derived from phencyclidine". Journal of Medicinal Chemistry. 24 (5): 496–9. doi:10.1021/jm00137a004. PMID 7241506.
- ^ Vignon J, Pinet V, Cerruti C, Kamenka JM, Chicheportiche R (April 1988). "[3H]N-[1-(2-benzo(b)thiophenyl)cyclohexyl]piperidine ([3H]BTCP): a new phencyclidine analog selective for the dopamine uptake complex". European Journal of Pharmacology. 148 (3): 427–36. doi:10.1016/0014-2999(88)90122-7. PMID 3384005.
- ^ Kamenka JM, et al. Substituted cyclic amines and pharmaceutical composition containing them. Patent US5248686, 28 September 1993
- ^ Wallach JV. Structure activity relationship (SAR) studies of arylcycloalkylamines as N-methyl-D-aspartate receptor antagonists. PhD. Thesis, University of the Sciences in Philadelphia, 19 Dec 2014.
- ^ Shulgin AT, Mac Lean DE (1976). "Illicit synthesis of phencyclidine (PCP) and several of its analogs". Clinical Toxicology. 9 (4): 553–60. doi:10.3109/15563657608988157. PMID 975751.
- ^ Sun S, Wallach J, Adejare A (2014). "Syntheses and N-methyl-D-aspartate receptor antagonist pharmacology of fluorinated arylcycloheptylamines". Medicinal Chemistry. Shariqah (United Arab Emirates). 10 (8): 843–52. doi:10.2174/1573406410666140428104444. PMID 24773376.
- ^ McQuinn RL, Cone EJ, Shannon HE, Su TP (December 1981). "Structure-activity relationships of the cycloalkyl ring of phencyclidine". Journal of Medicinal Chemistry. 24 (12): 1429–32. doi:10.1021/jm00144a011. PMID 7310819.
- ^ Eaton TA, Houk KN, Watkins SF, Fronczek FR (April 1983). "Geometries and conformational processes in phencyclidine and a rigid adamantyl analogue: variable-temperature NMR, X-ray crystallographic, and molecular mechanics studies". Journal of Medicinal Chemistry. 26 (4): 479–86. doi:10.1021/jm00358a005. PMID 6834381.
- ^ Gerhard O, Eberhard E. 4-amino-piperidines. US3311624A
- ^ Moriarty RM, Enache LA, Zhao L, Gilardi R, Mattson MV, Prakash O (February 1998). "Rigid phencyclidine analogues. Binding to the phencyclidine and sigma 1 receptors". Journal of Medicinal Chemistry. 41 (4): 468–77. doi:10.1021/jm970059p. PMID 9484497.
Further reading[]
- Morris H, Wallach J (2014). "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis. 6 (7–8): 614–32. doi:10.1002/dta.1620. PMID 24678061.
External links[]
- Arylcyclohexylamines
- General anesthetics
- NMDA receptor antagonists
- Chemical classes of psychoactive drugs