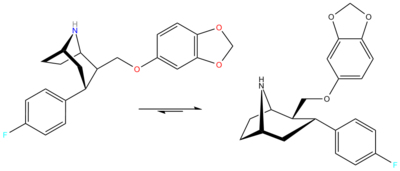
RTI-274
2β-([3,4-Methylenedioxy)phenoxy]methyl)-3α-(4-fluorophenyl)nortropane
Formula C 21 H 22 F N O 3 Molar mass −1 3D model (JSmol )
c4cc1OCOc1cc4OCC2C(c3ccc(F)cc3)CC5NC2CC5
RTI(-4229 )-274 , or 2β-((3,4-Methylenedioxyphenoxy)methyl)-3α-(4-fluorophenyl)nortropane is a phenyltropane homologue of paroxetine developed by the group led by F Ivy Carroll in the 1990s.[1]
Introduction [ ] Very few esters of phenyltropanes are actually known to have been reported.
NS2330 and NS2359 both have α,β stereochemistry.
NS2214 appears to have been abandoned now, RTI-336 was their latest compound.
RTI decided that they wanted to make all 8 stereoisomers of the phenyltropane paroxetine homolog.[1]
MAT IC50 (nM) Nor/tropane-Paroxetine Hybrids
Compound
[3 H]CFT
[3 H]Paroxetine
[3 H]Nisoxetine
Paroxetine
? → 623
? → 0.28
? → 535
R
"β,β"
308 → 835
294 → 480
5,300 → 37,400
α,β
172 → 142
52.9 → 90
26,600 → 2,500
β,α
3.01 → 3.86
422 → 5.62
123 → 14.4
S
"β,β"
1,050 → 1,210
88.1 → 424
27,600 → 17,300
α,β
1,500 → 27.6
447→ 55.8
2,916 → 1,690
β,α
298 → 407
178 → 19
12,400 → 1,990
N -demethylating the S -α,β (1S,2S,3R) isomer resulted in a 54-fold increase in DAT IC50 .In the case of nocaine it is understood that the SR enantiomer is the one that should be demethylated if it is wanted to improve DAT affinity.
That is the same enantiomer that is used in the production of paroxetine.
Skeletal rearrangement [ ]
Four years later some unrelated authors cited a skeletal rearrangement accounts for this.[2] Diagram [dead link
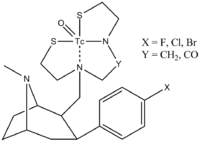
Notice that they are not only interested in ethers, but nitrogen containing Nu's ("")[3]
The metal is called "Technetium " and is bound by a chelating agent.
The authors state that at first the acid is halogenated, the amide is prepared, and reduced.
Erratum [ ] (a) (1) 1-chloroethyl chloroformate, 1,2-dichloroethane, reflux; (2) MeOH reflux; (b) p -toluenesulfonyl chloride,
triethylamine; (c) LiAlH4 , THF, rt; (d) trifluoromethanesulfonic anhydride, pyridine, CH2 Cl2 ; (e) Na, sesamol, THF; (f) 5% Na/Hg amalgam, Na2 HPO4 , MeOH.
MAT IC50 (Ki ) N -Methyl → De-methyl
Compound [3 H]CFT [3 H]Nisoxetine [3 H]paroxetine
R -β,β? → 3
? → 2 (0.2)
? → 6 (4)
S -β,β? → ?
? → ? (?)
? → ? (?)
R -"nonane"308 → 835
294 (27) → 480 (44)
5,300 (3200) → 37,400 (22,500)
S -"nonane"1050 → 1210
88 (8) → 424 (39)
27,600 (16,600) → 17,300 (10,400)
To solve the problem of the unexpected aza-bicyclo[3.2.2]nonane rearrangement product, the original synthesis had to be modified as follows;[4] WIN 35428 was N-demethylated and then the NH amine was reacted with a suitable protecting group so that N is no longer nucleophilic . In their case they used a tosyl.[3]
See also [ ] References [ ]
^ a b Keverline-Frantz KI, Boja JW, Kuhar MJ, Abraham P, Burgess JP, Lewin AH, Carroll FI (January 1998). "Synthesis and ligand binding of tropane ring analogues of paroxetine". Journal of Medicinal Chemistry . 41 (2): 247–57. doi :10.1021/jm970669p . PMID 9457247 . ^ Ogier L, Turpin F, Baldwin RM, Riché F, Law H, Innis RB, Tamagnan G (May 2002). "Rearrangement of a mesylate tropane intermediate in nucleophilic substitution reactions. Synthesis of aza-bicyclo[3.2.1]octane and aza-bicyclo[3.2.2]nonane ethers, imides, and amines". The Journal of Organic Chemistry . 67 (11): 3637–42. doi :10.1021/jo010973x . PMID 12027674 . ^ a b Singh S (March 2000). "Chemistry, design, and structure-activity relationship of cocaine antagonists". Chemical Reviews . 100 (3): 925–1024. doi :10.1021/cr9700538 . PMID 11749256 . S2CID 36764655 . ^ Runyon SP, Burgess JP, Abraham P, Keverline-Frantz KI, Flippen-Anderson J, Deschamps J, et al. (April 2005). "Synthesis, structural identification, and ligand binding of tropane ring analogs of paroxetine and an unexpected aza-bicyclo[3.2.2]nonane rearrangement product". Bioorganic & Medicinal Chemistry . 13 (7): 2439–49. doi :10.1016/j.bmc.2005.01.046 . PMID 15755646 .
Phenyltropanes (classifications )
2-Carboxymethyl Esters
Troparil WIN 35428 RTI-31 RTI-32 RTI-51 RTI-55 RTI-83 (3,4-Disubstituted Phenyl)-tropanes
Dichloropane RTI-112 RTI-353 Arylcarboxy Carboxyalkyl Acyl β,α Stereochemistry α,β Stereochemistry
Brasofensine Tesofensine NS2359 Heterocycles: 3-Substituted-isoxazol-5-yl Heterocycles: 3-Substituted-1,2,4-oxadiazole N-alkyl N-replaced (S,O,C) Irreversible Nortropanes (N-demethylated)
Stimulants
Adamantanes
Adapromine Amantadine Bromantane Memantine Rimantadine Adenosine antagonists Alkylamines
Cyclopentamine Cypenamine Cyprodenate Heptaminol Isometheptene Levopropylhexedrine Methylhexaneamine Octodrine Propylhexedrine Tuaminoheptane Ampakines Arylcyclohexylamines Benzazepines
6-Br-APB SKF-77434 SKF-81297 SKF-82958 Cathinones Cholinergics Convulsants Eugeroics Oxazolines Phenethylamines Phenylmorpholines Piperazines
2C-B-BZP 3C-PEP BZP CM156 DBL-583 GBR-12783 GBR-12935 GBR-13069 GBR-13098 GBR-13119 MeOPP MBZP oMPP Vanoxerine Piperidines
1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine 2-Benzylpiperidine 2-Methyl-3-phenylpiperidine 3,4-Dichloromethylphenidate 4-Benzylpiperidine 4-Fluoromethylphenidate 4-Methylmethylphenidate Desoxypipradrol Difemetorex Diphenylpyraline Ethylnaphthidate Ethylphenidate Methylnaphthidate Isopropylphenidate JZ-IV-10 Methylphenidate (Dexmethylphenidate )Nocaine Phacetoperane Pipradrol Propylphenidate SCH-5472 Pyrrolidines Racetams
Oxiracetam Phenylpiracetam Phenylpiracetam hydrazide Tropanes Tryptamines Others ATC code : N06B
D1 -like
Agonists
Ergolines : Cabergoline CY-208,243 Dihydroergocryptine Lisuride Pergolide Terguride Phenethylamines : Deoxyepinephrine (N-methyldopamine, epinine) Dopexamine Etilevodopa Ibopamine L -DOPA (levodopa)Melevodopa L -PhenylalanineL -TyrosineOthers : A-68930 Apomorphine Nuciferine PF-6649751 Propylnorapomorphine Rotigotine SKF-89,145 Stepholidine Tavapadon Tetrahydropalmatine PAMs Antagonists
Typical antipsychotics : Butaclamol Chlorpromazine Chlorprothixene Flupentixol (flupenthixol) (+melitracen )Fluphenazine Loxapine Perphenazine (+amitriptyline )Thioridazine Thiothixene Trifluoperazine (+tranylcypromine )Zuclopenthixol Atypical antipsychotics : Asenapine Clorotepine Clotiapine Clozapine DHA-clozapine Fluperlapine Iloperidone Norclozapine Olanzapine (+fluoxetine )Paliperidone Quetiapine Risperidone Zicronapine Ziprasidone Zotepine Others : Ecopipam EEDQ Metitepine (methiothepin) Perlapine SCH-23390
D2 -like
Agonists
Adamantanes : Amantadine Memantine Rimantadine Aminotetralins : 5-OH-DPAT 7-OH-DPAT 8-OH-PBZI Rotigotine UH-232 Ergolines : Bromocriptine Cabergoline Chanoclavine Dihydroergocryptine Epicriptine Ergocornine Lergotrile Lisuride LSD Pergolide Terguride Phenethylamines : Deoxyepinephrine (N-methyldopamine, epinine) Dopexamine Etilevodopa Ibopamine L -DOPA (levodopa)L -PhenylalanineL -TyrosineMelevodopa Atypical antipsychotics : Alentemol (U-66444B) Aripiprazole (+sertraline )Aripiprazole lauroxil Bifeprunox Brexpiprazole Brilaroxazine Cariprazine F-15063 Lumateperone Norclozapine Others : 3-PPP A-412997 ABT-670 ABT-724 Adrafinil Aplindore Apomorphine Arketamine Armodafinil BP-897 Captodiame CP-226,269 Dizocilpine Esketamine Flibanserin Ketamine Mesulergine Modafinil OSU-6162 Pardoprunox PD-128,907 PD-168,077 PF-219,061 PF-592,379 Phencyclidine Piribedil Pramipexole Preclamol Propylnorapomorphine Pukateine Quinagolide Quinelorane Quinpirole RDS-127 Ro10-5824 Ropinirole Roxindole Salvinorin A SKF-83,959 Sumanirole Talipexole Umespirone WAY-100,635 Antagonists
Antiemetics/gastroprokinetics/sedatives : Aceprometazine AS-8112 Alimemazine Alizapride Benzquinamide Bromopride Clebopride Deudomperidone Domperidone Eticlopride Hydroxyzine Itopride Metoclopramide Metopimazine Promethazine Thiethylperazine Trimethobenzamide Antidepressants : Amoxapine Nefazodone Opipramol Propiomazine Trimipramine Others : 3-PPP Azapride Bromerguride Bromocriptine Buspirone Desmethoxyfallypride EEDQ F-15063 Fallypride Fananserin Fenfluramine Iodobenzamide L-741,626 L-745,870 Levofenfluramine Metergoline Metitepine (methiothepin) N-Methylspiperone Nafadotride Nuciferine PNU-99,194 Pridopidine Raclopride Sarizotan SB-277,011-A Sonepiprazole Spiroxatrine Stepholidine Terguride Tetrahydropalmatine Tiapride UH-232 Yohimbine
See also: Receptor/signaling modulators Adrenergics Serotonergics Monoamine reuptake inhibitors Monoamine releasing agents Monoamine metabolism modulators Monoamine neurotoxins