Ampelopsin
| |
| Names | |
|---|---|
| IUPAC name
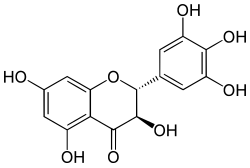
(2R,3R)-3,3′,4′,5,5′,7-Hexahydroxyflavan-4-one
| |
| Preferred IUPAC name
(2R,3R)-3,5,7-Trihydroxy-2-(3,4,5-trihydroxy)-2,3-dihydro-4H-1-benzopyran-4-one | |
| Other names
Dihydromyricetin, Ampeloptin,(+)-Ampelopsin,(+)-Dihydromyricetin
| |
| Identifiers | |
CAS Number
|
|
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
CompTox Dashboard (EPA)
|
|
InChI
| |
SMILES
| |
| Properties | |
Chemical formula
|
C15H12O8 |
| Molar mass | 320.253 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Ampelopsin, also known as dihydromyricetin and DHM, when purported as an effective ingredient in supplements and other tonics, is a flavanonol, a type of flavonoid. It is found in the Ampelopsis species japonica, megalophylla, and grossedentata; Cercidiphyllum japonicum; Hovenia dulcis; Rhododendron cinnabarinum; some Pinus species; and some Cedrus species,[1] as well as in Salix sachalinensis.[2]
Hovenia dulcis has been used in traditional Japanese, Chinese, and Korean medicines to treat fever, parasitic infection, as a laxative, and a treatment of liver diseases, and as a hangover treatment.[3] Methods have been developed to extract ampelopsin from it at large scales, and laboratory research has been conducted with the compound to see if it might be useful as a drug in any of the conditions for which the parent plant has been traditionally used.[3]
Scientific peer-reviewed research[]
In a trial of 60 patients with "nonalcoholic fatty liver disease," dihydromyricetin improved glucose and lipid metabolism and yielded potentially beneficial anti-inflammatory effects.[4]
In a study wherein the subjects were rats, researchers demonstrated "pharmacological properties of dihydromyricetin consistent with those expected to underlie successful medical treatment of alcohol use disorders; therefore dihydromyricetin is a therapeutic candidate."[5]
"Structurally, due to the highly hydrophilic character, dihydromyricetin shows poor bioavailability and significantly limits its potential medicinal applications."[6] "In pharmacology, bioavailability [...] is a subcategory of absorption and is the fraction (%) of an administered drug that reaches the systemic circulation."[7][circular reference]
As they pertain to dihydromyricetin, additional research is required before claims of human efficacy and application, necessary dosage, and solutions to poor bioavailability, are met with scientific validation.
References[]
- ^ Zhou, Jiaju; Xie, Guirong; Yan, Xinjian (2011-02-21). Encyclopedia of Traditional Chinese Medicines – Molecular Structures, Pharmacological Activities, Natural Sources and Applications: Vol. 1: Isolated Compounds A-C. Springer Science & Business Media. ISBN 978-3-642-16735-5.
- ^ Tahara, Satoshi (June 23, 2007). "A Journey of Twenty-Five Years through the Ecological Biochemistry of Flavonoids". Bioscience, Biotechnology, and Biochemistry. 71 (6): 1387–1404. doi:10.1271/bbb.70028. ISSN 0916-8451. PMID 17587669. S2CID 35670587.
- ^ a b Hyun, Tae; Eom, Seung; Yu, Chang; Roitsch, Thomas (2010). "Hovenia dulcis– An Asian Traditional Herb". Planta Medica. 76 (10): 943–949. doi:10.1055/s-0030-1249776. ISSN 0032-0943. PMID 20379955.
- ^ Chen, Shihui; Zhao, Xiaolan; Wan, Jing; Ran, Li; Qin, Yu; Wang, Xiaofang; Gao, Yanxiang; Shu, Furong; Zhang, Yong; Liu, Peng; Zhang, Qianyong; Zhu, Jundong; Mi, Mantian (2015). "Dihydromyricetin improves glucose and lipid metabolism and exerts anti-inflammatory effects in nonalcoholic fatty liver disease: A randomized controlled trial". Pharmacological Research. 99: 74–81. doi:10.1016/j.phrs.2015.05.009. ISSN 1043-6618. PMID 26032587.
- ^ Yi Shen; A. Kerstin Lindemeyer; Claudia Gonzalez; Xuesi M. Shao; Igor Spigelman; Richard W. Olsen & Jing Liang (2012). "Dihydromyricetin as a novel anti-alcohol intoxication medication". The Journal of Neuroscience. 32 (1): 390–401. doi:10.1523/JNEUROSCI.4639-11.2012. PMC 3292407. PMID 22219299.
- ^ Li, Hongliang; Li, Qisheng; Liu, Zhaowen; Yang, Kai; Chen, Zhixi; Cheng, Qilai; Wu, Longhuo (2017). "The Versatile Effects of Dihydromyricetin in Health". Evidence-Based Complementary and Alternative Medicine. 2017: 1053617. doi:10.1155/2017/1053617. ISSN 1741-427X. PMC 5602609. PMID 28947908.
- ^ "Bioavailability - Wikipedia". en.m.wikipedia.org. Retrieved 2020-04-28.
- Flavanonols
- Pyrogallols
- Resorcinols
- GABAA receptor positive allosteric modulators