An amyl alcohol is any of eight alcohols with the formula C5 H12 O.[1] amyl alcohols (also called amyl alcohol) can be obtained from fusel alcohol . Amyl alcohol is used as a solvent and in esterification , by which is produced amyl acetate and other important products. The name amyl alcohol without further specification applies to the normal (straight-chain) form, 1-pentanol .[2]
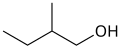
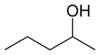
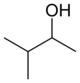
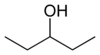
These are the 8 alcohols that are structural isomers with molecular formula C5 H12 O:
Amyl alcohol isomers
1-pentanol
primary
Pentan-1-ol
138.5
2-methyl-1-butanol
primary
2-Methylbutan-1-ol
128.7
3-methyl-1-butanol
primary
3-Methylbutan-1-ol
131.2
2,2-dimethyl-1-propanol
primary
2,2-Dimethylpropan-1-ol
113.1
2-pentanolsec -amyl alcohol
secondary
Pentan-2-ol
118.8
3-methyl-2-butanolsec -isoamyl alcohol
secondary
3-Methylbutan-2-ol
113.6
3-Pentanol
secondary
Pentan-3-ol
115.3
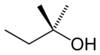
2-methyl-2-butanoltert -amyl alcohol
tertiary
2-Methylbutan-2-ol
102
Three of these alcohols, 2-methyl-1-butanol, 2-pentanol, and 3-methyl-2-butanol (methyl isopropyl carbinol), are therefore optically active .
The most important amyl alcohol is isoamyl alcohol , the chief one generated by fermentation in the production of alcoholic beverages and a constituent of fusel oil . The other amyl alcohols may be obtained synthetically.
Notes [ ] show Alcohols
By consumption
Alcohols found inalcoholic drinks
1-Propanol 2-Methyl-1-butanol Ethanol Isoamyl alcohol Isobutanol Phenethyl alcohol tert -Amyl alcoholtert -Butyl alcoholTryptophol Medical alcohol Toxic alcohols
Isopropyl alcohol Methanol
Primary (1°)
Methanol
4-Methylcyclohexanemethanol Aminomethanol Cyclohexylmethanol Methoxymethanol Methylazoxymethanol Trifluoromethanol Ethanol
1-Aminoethanol 2,2,2-Trichloroethanol 2,2,2-Trifluoroethanol 2-(2-Ethoxyethoxy)ethanol 2-(2-Methoxyethoxy)ethanol 2-(2-Methoxyethoxy)ethanol 2-Butoxyethanol 2-Chloroethanol 2-Ethoxyethanol 2-Fluoroethanol 2-Mercaptoethanol 2-Methoxyethanol Aminoethylethanolamine Diethylethanolamine Dimethylethanolamine Ethanol Ethanolamine N ,N -DiisopropylaminoethanolN -MethylethanolaminePhenoxyethanol Tribromoethanol Butanol
2-Methyl-1-butanol Isobutanol n -Butanol Straight-chain1 — C9
Methanol Ethanol 1-Propanol 1-Butanol 1-Pentanol 1-Hexanol 1-Heptanol 1-Octanol (capryl)1-Nonanol (pelargonic) Straight-chain10 — C19
1-Decanol (capric)1-Undecanol (hendecyl)1-Dodecanol (lauryl)1-Tridecanol 1-Tetradecanol (myristyl)1-Pentadecanol 1-Hexadecanol (cetyl / palmityl)1-Heptadecanol 1-Octadecanol (stearyl)1-Nonadecanol Straight-chain20 — C29
1-Icosanol (arachidyl)1-Docosanol (behenyl)1-Tetracosanol (lignoceryl)1-Hexacosanol (ceryl)1-Heptacosanol 1-Octacosanol (cluytyl / montanyl)1-Nonacosanol Straight-chain30 — C39
1-Triacontanol (melissyl / myricyl)1-Dotriacontanol (lacceryl) (geddyl)
Straight-chain40 — C49
2-Ethylhexanol Allyl alcohol Anisyl alcohol Benzyl alcohol Cinnamyl alcohol Crotyl alcohol Furfuryl alcohol Isoamyl alcohol Neopentyl alcohol Nicotinyl alcohol Perillyl alcohol Phenethyl alcohol Propargyl alcohol Salicyl alcohol Tryptophol Vanillyl alcohol Veratrole alcohol Secondary
1-Phenylethanol 2-Butanol 2-Heptanol 3-Heptanol 2-Hexanol 3-Hexanol 3-Methyl-2-butanol 2-Nonanol 2-Octanol 2-Pentanol 3-Pentanol Cyclohexanol Cyclopentanol Cyclopropanol Diphenylmethanol Isopropanol Pinacolyl alcohol Pirkle's alcohol Propylene glycol methyl ether Tertiary
2-Methyl-2-pentanol 3-Methyl-3-pentanol Diacetone alcohol Ethchlorvynol Methylpentynol Nonafluoro-tert -butyl alcohol tert -Amyl alcoholtert -Butyl alcoholTriphenylethanol Triphenylmethanol Hydric alcohols
Monohydric alcohols
Methanol Ethanol Isopropanol Dihydric alcohols Trihydric alcohols
Amyl alcohols
2,2-Dimethylpropan-1-ol 2-Methylbutan-1-ol 2-Methylbutan-2-ol 3-Methylbutan-1-ol 3-Methylbutan-2-ol Pentan-1-ol Pentan-2-ol Pentan-3-ol Aromatic alcohols
Benzyl alcohol 2,4-Dichlorobenzyl alcohol 3-Nitrobenzyl alcohol Branched andfatty alcohols
tert-Butyl alcohol tert-Amyl alcohol 3-Methyl-3-pentanol Oleyl alcohol Sugar alcohols
C2 — C7
Ethylene glycol (C2 )Glycerol (C3 )Erythritol (C4 )Threitol (C4 )Arabitol (C5 )Ribitol (C5 )Xylitol (C5 )Mannitol (C6 )Sorbitol (C6 )Galactitol (C6 )Iditol (C6 )Volemitol (C7 ) Deoxy sugar Cyclic sugar Glycylglycitols
Maltitol Lactitol Isomalt
Terpene alcohols
Monoterpene Diterpene
Dialcohols
1,4-Butanediol 1,5-Pentanediol 2-Methyl-2-propyl-1,3-propanediol Diethylpropanediol Ethylene glycol Catechol Fluoroalcohols
1,3-Difluoro-2-propanol 2,2,2-Trifluoroethanol 2-Fluoroethanol Nonafluoro-tert -butyl alcohol Trifluoromethanol Preparations Reactions
Category
show GABA A receptor positive modulatorsAlcohols
Butanol Chloralodol Chlorobutanol (cloretone) Ethanol (alcohol) (alcoholic drink )Ethchlorvynol Isobutanol Isopropanol Menthol Methanol Methylpentynol Pentanol Petrichloral Propanol tert -Butanol (2M2P)tert -Pentanol (2M2B)Tribromoethanol Trichloroethanol Triclofos Trifluoroethanol Barbiturates Benzodiazepines Carbamates Flavonoids
Ampelopsin (dihydromyricetin) Apigenin Baicalein Baicalin Catechin EGC EGCG Hispidulin Luteolin Skullcap constituents (e.g., baicalin )Wogonin Imidazoles Kava constituents
Desmethoxyyangonin Kavain Methysticin Yangonin Monoureides Neuroactive steroids Nonbenzodiazepines Phenols
Fospropofol Propofol Thymol Piperidinediones Pyrazolopyridines Quinazolinones Volatiles /gases
Acetone Acetophenone Acetylglycinamide chloral hydrate Aliflurane Benzene Butane Butylene Centalun Chloral Chloral betaine Chloral hydrate Chloroform Cryofluorane Desflurane Dichloralphenazone Dichloromethane Diethyl ether Enflurane Ethyl chloride Ethylene Fluroxene Gasoline Halopropane Halothane Isoflurane Kerosine Methoxyflurane Methoxypropane Nitric oxide Nitrogen Nitrous oxide Norflurane Paraldehyde Propane Propylene Roflurane Sevoflurane Synthane Teflurane Toluene Trichloroethane (methyl chloroform) Trichloroethylene Vinyl ether Others/unsorted
3-Hydroxybutanal Avermectins (e.g., ivermectin )Bromide compounds (e.g., lithium bromide , potassium bromide , sodium bromide )Carbamazepine Chloralose Chlormezanone Clomethiazole DEABL Dihydroergolines (e.g., dihydroergocryptine , , dihydroergotamine , ergoloid (dihydroergotoxine) )Efavirenz Etazepine Etifoxine Fenamates (e.g., flufenamic acid , mefenamic acid , niflumic acid , tolfenamic acid )Fluoxetine Flupirtine Hopantenic acid Lanthanum Lavender oil Lignans (e.g., 4-O-methylhonokiol , honokiol , magnolol , obovatol )Loreclezole Menthyl isovalerate (validolum) Monastrol Niacin Niacinamide Org 25,435 Phenytoin Propanidid Retigabine (ezogabine) Safranal Seproxetine Stiripentol (e.g., sulfonmethane (sulfonal) , tetronal , trional )
Terpenoids (e.g., borneol )Topiramate Valerian constituents (e.g., isovaleric acid , isovaleramide , valerenic acid , )Unsorted benzodiazepine site positive modulators: α-Pinene See also: Receptor/signaling modulators • GABA receptor modulators • GABA metabolism/transport modulators
show Authority control General Other