Ethylone Pregnancy Routes of Oral, nasal, IV Legal status
DE Anlage I (Authorized scientific use only) UK :Class B
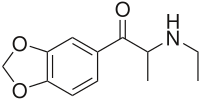
show (RS )-1-(1,3-benzodioxol-5-yl)-2-(ethylamino)propan-1-one
CAS Number PubChem CID ChemSpider UNII CompTox Dashboard (EPA ) Formula C 12 H 15 N O 3 Molar mass −1 3D model (JSmol ) Chirality Racemic mixture show CC(NCC)C(=O)c1ccc2OCOc2c1
show InChI=1S/C12H15NO3/c1-3-13-8(2)12(14)9-4-5-10-11(6-9)16-7-15-10/h4-6,8,13H,3,7H2,1-2H3
N Key:MJEMIOXXNCZZFK-UHFFFAOYSA-N
N N Y (what is this?)
Ethylone , also known as 3,4-methylenedioxy-N -ethylcathinone (MDEC , βk-MDEA ), is a recreational designer drug classified as an entactogen , stimulant , and psychedelic of the phenethylamine , amphetamine , and cathinone chemical classes . It is the β-keto analogue of MDEA ("Eve"). Ethylone has only a short history of human use and is reported to be less potent than its relative methylone .[citation needed In the United States, it began to be found in cathinone products in late 2011.[1]
Very little data exists about the pharmacological properties, metabolism, and toxicity of ethylone, and although several ethylone-related deaths have been reported, the cause of death was not due to ingestion of ethylone.[1]
Pharmacokinetics [ ] Analysis of human and rat urine for the metabolites of bk-amphetamines suggested that ethylone was degraded in the following metabolic steps:[2]
N-deethylation to the primary amine .
Reduction of the keto moiety to the respective alcohol .Legal Status [ ] As of October 2015 Ethylone is a controlled substance in China.[3]
See also [ ] References [ ] External links [ ] show Empathogens/entactogens
Phenylalkyl-
Unfused benzene ring: 3-CMA 4-CAB 4-FA 4-MA 4-MMA 4-FMA 4-MTA 4,4'-DMAR Ariadne Metaescaline MMA PMA PMEA PMMA mMMA Benzodioxine: EDMA Benzodioxoles: Phenethylamine: { Lophophine }Amphetamines: { 2-Methyl-MDA
2,3-MDA 3,4-MDA (tenamfetamine) 5-Methyl-MDA 6-Methyl-MDA DiFMDA DMMDA DMMDA-2 EIDA MDEA MDMA (midomafetamine) MDMOH MDOH MMDA MMDA-2 MMDMA N-t-BOC-MDMA }1-Phenylbutan-2-amines: { BDB
EBDB MBDB }Phentermines: { MDMP
MDPH }Benzofurans and dihydrobenzofurans: 5-APB 5-APDB 5-EAPB 5-MAPB 5-MAPDB 5-MBPB 6-APB 6-APDB 6-EAPB 6-MAPB 6-MAPDB IBF5MAP Indanes: 5-APDI 5-MAPDI Indoles: 5-IT 6-API Naphthalenes: Methamnetamine Naphthylaminopropane Tetralin: 6-APT Cyclized phenyl-
Aminoindanes: 5-IAI AMMI ETAI MDAI MDMAI MMAI MEAI NM-2-AI TAI Aminotetralins: 6-CAT MDAT MDMAT Cathinones
3-MMC 4-EMC Brephedrone Butylone Ethylone Eutylone Flephedrone Mephedrone Methedrone Methylone TH-PVP Tryptamines Chemical classes
Substituted amphetamine Sub. benzofuran Sub. cathinone Sub. MDPEA Sub. PEA Sub. tryptamine
show Hallucinogens
Psychedelics (5-HT2A
Benzofurans
2C-B-FLY 2CBFly-NBOMe 5-MeO-BFE 5-MeO-DiBF Bromo-DragonFLY F-2 F-22 TFMFly Lyserg‐
1B-LSD 1cP-LSD 1P-ETH-LAD 1P-LSD 1V-LSD 2-Butyllysergamide 3-Pentyllysergamide AL-LAD ALD-52 BU-LAD Diallyllysergamide Dimethyllysergamide ECPLA Ergometrine ETH-LAD IP-LAD LAE-32 LPD-824 LSA LSD LSD-Pip LSH LSM-775 LSZ Methylergometrine MIPLA Methysergide MLD-41 PARGY-LAD PRO-LAD Phenethyl‐
Piperazines Tryptamines
alpha -alkyltryptamines
4,5-DHP-α-MT 5-MeO-α-ET 5-MeO-α-MT α-ET α-MT x -DALT
(Daltacetin) 4-AcO-DALT 5-MeO-DALT DALT x -DET
(Ethacetin) 4-AcO-DET (Ethocin) 4-HO-DET 5-MeO-DET (T-9) DET (Ethocybin) 4-PO-DET x -DiPT
(Ipracetin) 4-AcO-DiPT (Iprocin) 4-HO-DiPT 5-MeO-DiPT DiPT x -DMT
4,5-DHP-DMT 2,N,N-TMT 4-AcO-DMT 4-HO-5-MeO-DMT 4-Propionyloxy-DMT 5-Bromo-DMT 5-MeO-DMT 5-N ,N -TMT 7,N,N-TMT α,N,N-TMT (Bufotenin) 5-HO-DMT DMT Norbaeocystin (Psilocin) 4-HO-DMT (Psilocybin) 4-PO-DMT x -DPT
(Deprocin) 4-HO-DPT 5-MeO-DPT (The Light) DPT Ibogaine-related
18-MAC 18-MC Coronaridine Ibogaine Ibogamine ME-18-MC Noribogaine Tabernanthine Voacangine x -MET
(Metocin) 4-HO-MET (Metocetin) 4-AcO-MET 5-MeO-MET MET x -MiPT
(Mipracetin) 4-AcO-MiPT (Miprocin) 4-HO-MiPT 5-Me-MiPT (Moxy) 5-MeO-MiPT MiPT Others
4-HO-DBT 4-HO-EPT 4-HO-McPT (Lucigenol) 4-HO-MPMI (Meprocin) 4-HO-MPT 5-MeO-EiPT 5-MeO-MALT 5-MeO-MPMI Aeruginascin Baeocystin DBT EiPT EPT MPT PiPT
Others
AL-38022A ALPHA Dimemebfe Efavirenz Glaucine Lorcaserin M-ALPHA RH-34 Also empathogens in general (e. g.: 5-APB , 5-MAPB , 6-APB and other substituted benzofurans ).
Dissociatives (NMDAR antagonists )
Arylcyclo‐
Ketamine-related PCP-related Others
Diarylethylamines Morphinans
Dextrallorphan Dextromethorphan Dextrorphan Racemethorphan Racemorphan Others
Deliriants (mAChR antagonists ) Others
Cannabinoids (CB1 agonists)
Natural
Salvinorin A THC (Dronabinol) THCV Synthetic
D2 agonists
Apomorphine Aporphine Bromocriptine Cabergoline Lisuride LSD Memantine Nuciferine Pergolide Phenethylamine Piribedil Pramipexole Ropinirole Rotigotine Salvinorin A Also indirect D2 agonists, such as dopamine reuptake inhibitors (cocaine , methylphenidate ), releasing agents (amphetamine , methamphetamine ), and precursors (levodopa ). GABAA
CI-966 Eszopiclone Ibotenic acid Muscimol (Amanita muscaria Zaleplon Zolpidem Zopiclone Inhalants (Mixed MOA )
Aliphatic hydrocarbons
Butane Gasoline Kerosene Propane Aromatic hydrocarbons
Ethers
Haloalkanes
Chlorofluorocarbons Chloroform κOR agonists
2-EMSB Alazocine Bremazocine Butorphan Butorphanol Cyclazocine Cyclorphan Cyprenorphine Diprenorphine Enadoline Herkinorin Heroin HZ-2 Ibogaine Ketazocine Levallorphan Levomethorphan Levorphanol LPK-26 Metazocine Morphine Nalbuphine Nalmefene Nalorphine Noribogaine Oxilorphan Pentazocine Phenazocine Proxorphan Racemethorphan Racemorphan Salvinorin A Spiradoline Tifluadom U-50488 U-69,593 Xorphanol Oneirogens
Calea zacatechichi Silene capensis Galantamine Others
Glaucine Isoaminile Noscapine Pukateine
show Stimulants
Adamantanes
Adapromine Amantadine Bromantane Memantine Rimantadine Adenosine antagonists
8-Chlorotheophylline 8-Cyclopentyltheophylline 8-Phenyltheophylline Aminophylline Caffeine CGS-15943 Dimethazan Istradefylline Paraxanthine SCH-58261 Theobromine Theophylline Alkylamines
Cyclopentamine Cypenamine Cyprodenate Heptaminol Isometheptene Levopropylhexedrine Methylhexaneamine Octodrine Propylhexedrine Tuaminoheptane Ampakines Arylcyclohexylamines Benzazepines
6-Br-APB SKF-77434 SKF-81297 SKF-82958 Cathinones
3-Fluoromethcathinone 3,4-DMMC 4-BMC 4-CMC 4-Methylbuphedrone 4-Methylcathinone 4-MEAP 4-Methylpentedrone Amfepramone Benzedrone Buphedrone Bupropion Butylone Cathinone Dimethylcathinone Ethcathinone Ethylone Flephedrone Hexedrone Isoethcathinone Mephedrone Methcathinone Methedrone Methylenedioxycathinone Methylone Mexedrone N-Ethylbuphedrone N-Ethylhexedrone Pentedrone Pentylone Phthalimidopropiophenone Cholinergics Convulsants Eugeroics
Adrafinil Armodafinil CRL-40,940 CRL-40,941 Fluorenol Modafinil Oxazolines
4-Methylaminorex Aminorex Clominorex Cyclazodone Fenozolone Fluminorex Pemoline Thozalinone Phenethylamines Phenylmorpholines
3-Fluorophenmetrazine Fenbutrazate Fenmetramide G-130 Manifaxine Morazone Morforex Oxaflozane PD-128,907 Phendimetrazine Phenmetrazine 2-Phenyl-3,6-dimethylmorpholine Pseudophenmetrazine Radafaxine Piperazines
2C-B-BZP 3C-PEP BZP CM156 DBL-583 GBR-12783 GBR-12935 GBR-13069 GBR-13098 GBR-13119 MeOPP MBZP oMPP Vanoxerine Piperidines
1-Benzyl-4-(2-(diphenylmethoxy)ethyl)piperidine 2-Benzylpiperidine 2-Methyl-3-phenylpiperidine 3,4-Dichloromethylphenidate 4-Benzylpiperidine 4-Fluoromethylphenidate 4-Methylmethylphenidate Desoxypipradrol Difemetorex Diphenylpyraline Ethylnaphthidate Ethylphenidate Methylnaphthidate Isopropylphenidate JZ-IV-10 Methylphenidate (Dexmethylphenidate )Nocaine Phacetoperane Pipradrol Propylphenidate SCH-5472 Pyrrolidines Racetams
Oxiracetam Phenylpiracetam Phenylpiracetam hydrazide Tropanes
4-fluorotropacocaine 4'-Fluorococaine Altropane (IACFT) Brasofensine CFT (WIN 35,428) β-CIT (RTI-55) Cocaethylene Cocaine Dichloropane (RTI-111) Difluoropine FE-β-CPPIT FP-β-CPPIT Ioflupane (123 I) Norcocaine PIT PTT RTI-31 RTI-32 RTI-51 RTI-112 RTI-113 RTI-120 RTI-121 (IPCIT) RTI-126 RTI-150 RTI-177 RTI-229 RTI-336 RTI-354 RTI-371 RTI-386 Salicylmethylecgonine Tesofensine Troparil (β-CPT, WIN 35,065-2) Tropoxane WF-23 WF-33 Tryptamines Others
2-MDP 3,3-Diphenylcyclobutanamine Amfonelic acid Amineptine Amiphenazole Atipamezole Atomoxetine Bemegride Benzydamine BTQ BTS 74,398 Centanafadine Ciclazindol Clofenciclan Cropropamide Crotetamide D-161 Desipramine Diclofensine Dimethocaine Efaroxan Etamivan Fenisorex Fenpentadiol Gamfexine Gilutensin GSK1360707F GYKI-52895 Hexacyclonate Idazoxan Indanorex Indatraline JNJ-7925476 Lazabemide Leptacline Lomevactone LR-5182 Mazindol Meclofenoxate Medifoxamine Mefexamide Methamnetamine Methastyridone Methiopropamine Naphthylaminopropane Nefopam Nikethamide Nomifensine O-2172 Oxaprotiline PNU-99,194 PRC200-SS Rasagiline Rauwolscine Rubidium chloride Setazindol Tametraline Tandamine Thiopropamine Thiothinone Trazium UH-232 Yohimbine ATC code : N06B